Why Does Nacl Not Conduct Electricity In Solid State
Explain why a salt which does not conduct electricity in the solid state becomes a good conductor in molten state. In the solid state however the ions are trapped in a lattice by electrostatic forces.
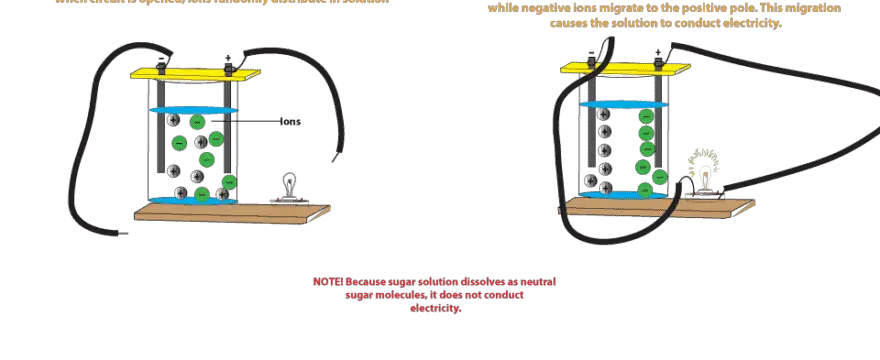
Why Does Salt Solution Conduct Electricity While Sugar Solution Doesn T
Answered Aug 14 2019 by priya12 -12624 points Although solid ionic compounds are made up of ions but they do not conduct electricity in solid state.

Why does nacl not conduct electricity in solid state. Explain NaCl is not a conductor of electricity in solid state whereas it does conduct electricity in aqueous solution as well as in molten state. Apne doubts clear karein ab Whatsapp par bhi. These free ions are responsible for conduction of electricity.
This means that the charged particles cannot move around freely and therefore salts do not conduct electrivity in their solid state. Hence due to the non availability of free ions or electrons in the solid crystal of Nacl which are responsible for the transfer of charges from one place to. However in the molten state ions in ionic compounds are free to flow and therefore molten sodium chloride can conduct electricity.
Asked Sep 6 2018 in. Sodium chloride is an ionic compound having sodium and chloride ions in its structure. N a C l is not a conductor of electricity in solid state whereas it does conduct electricity in aqueous solution as well as in molten state.
Ionic compounds do not conduct electricity in solid state but they conduct electricity in aqueous solution and in molten stateHence this property is shown by sodium chloride. It needs to be either melted molten or dissolved in a solution ie. This is because the electrons in a solid state wont be free to move to either the Cathode -ve or to the Anode ve.
3182018 Click here to get an answer to your question Why does NaCl conduct electricity in solid state but not in molten state. Figure 219 Ionic solid melting to produce ions that are free to move. 2922016 When sodium chloride is in its solid state there are no available electrons that may be shifted to facilitate the conduction of electricity.
Chemistry tutor 11114 Views. Reason In solid N a C l the ions have fixed positions in the lattice structure and there are no free ions but in aqueous solution or molten state the ions can move freely and thus conduct electricity. When sodium chloride is in the molten or aqueous state Its electrostatic force of attraction between the molecules is less and thus it easily break into ions to conduct electricity.
That is why solid ionic compounds will not conduct. 2912018 In solid NaCl the movement of ions is not possible due to its rigid structure but in aqueous solution or molten state the ions can move freely. Answered by Olavo M.
1492015 The particles of a substance that is in a solid state of matter move only slightly. This is because in the solid ionic compound the ions are held together in fixed positions by strong electrostatic forces and cannot move freely. Answered by Thomas D.
3032020 159 viewsApril 1 2020 0 Question2608K March 30 2020 25 Comments Why does NaCl solution conduct electricity while solid NaCl. Delocalized freely moving electrons in the conduction band are what makes solids conductive. 872012 But in NaCl in solid form Na positive ion and Cl negative ion are held together by strong electrostatic force and there is no freely moving ion in NaCl in solid state therefore NaCl does not.
3182008 NaCl common salt is solid in state and solid ions or compounds dont conduct electricity. If you apply a lot of energy to them to where they can start to conduct then you further ionize the solid and no longer have NaCl. I will move the question to physics forum as it.
192016 Explain why sodium chloride does not conduct electricity when it is a solid. Comparing NaCl with other ionic compounds electrodes. Therefore ionic compounds like sodium chloride NaCl do not conduct electricity well when they are solids.
1322015 The lower levels are all filled up and so if you apply a little bit of energy those electrons the is no where for them to go and they stay put. In the solid state ionic compounds such as sodium chloride have their ions fixed in position and therefore these ions cannot move so solid ionic compounds cannot conduct electricity. 652017 If what you mean is that NaCl crystal has no free moving ions nor free moving electrons I would accept it.
2032020 Explain why a salt which does not conduct electricity in the solid state becomes a good conductor in molten state. 24112018 It is because when sodium chloride is in solid state the electrostatic force between the molecules is high which does not allow it to break into ions.

Explain The Following A Sodium Chloride Is An Ionic Compound

8 Explain Why A Sodium Chloride Does Not Conduct Electri Scholr
Komentar
Posting Komentar