Na Orbital Diagram Of Valence Configuration
Symbol Total number of Orbital Diagram. Draw the valence dot diagrams for the following elements.
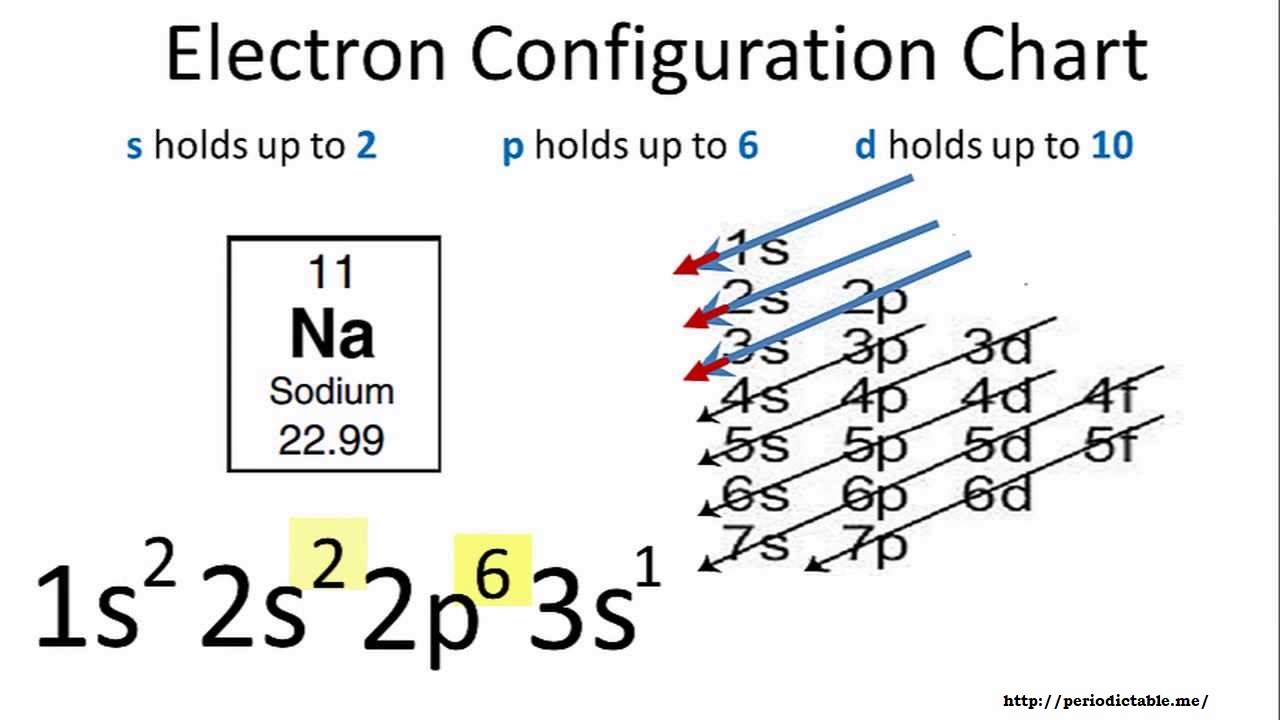
Sodium Electron Configuration Na With Orbital Diagram
Write orbital diagrams for the valence electrons and indicate the number of unpaired electrons for each element.

Na orbital diagram of valence configuration. Orbital Diagrams and Electron Configuration. Start studying Orbital Diagrams Electron Configuration Valence electrons. The nex six electrons will go in the 2p orbital.
Apart from that one more thing is unique about the element ie nitrogen can have either one of 3 or 5 valence electrons. Choose the valence orbital diagram that represents the ground state of Zn. Identify the element that has a ground state electronic configuration of Kr5s24d5.
The number of valence electrons impacts on their chemical properties and the specific ordering and properties of the orbitals are important in physics so many students have to get to grips with the basics. The electrons in an atom occupy distinct principal energy levels. Since 1s can only hold two electrons the next 2 electrons for sodium go in the 2s orbital.
Na 1s1 1s 22s 2p63s1 1s22s22p6 1s 22s 2p5 1s 22s 2p4 1s22s22p3 1s22s22p2 1s22s1 1s2 NOT CORRECT. For instance sodiums electron configuration can be written Ne3s1 essentially its the same as neon but with one more electron in the 3s orbital. A Br b Kr c Na.
To be located in. Use these elements to answer the questions below S Cl Al Na a Increasing ionization energy b decreasing electronegativity. The orbital diagram of an atom is a pictorial representation of all of its electrons.
Givenchemical species Asked formolecular orbital energy-level diagram valence electron configuration bond order and stability Strategy. Learn vocabulary terms and more with flashcards games and other study tools. May 08 2020 Note that electron configurations can be written in a sort of shorthand by using noble gasses the elements in group 18 to stand in for the orbitals at the start of the configuration.
Thus sodium ion Na has eight valence electrons. Short-hand Electron configuration. Learning Check Full Electron Configuration Element Condensed Electron Configuration Partial Orbital Diagram 1s 22s 2p63s1 Na Ne3s1 1s 22s 2p63s Mg Ne3s2 1s 22s 2p63s23p4 S Ne3s23p4 1s 22s 2p63s23p5 Cl Ne3s23p5 2.
There is a major exception to the normal order of electron configuration at Cr 24 and Cu 29. Shorthand Configuration S 16e-Core Electrons Valence Electrons S 16e-Ne3s2 3p4. It turns out that the energy the electron configuration that is half-filled 4s 1 3d 5 and filled orbital 4s 1 3d 10 has lower energy than the typical filling order 4s 2 3d 4 and 4s 2 3d 9This pattern is followed in the 5 th row with Mo.
Write out the complete electron configuration. In writing the electron configuration for sodium the first two electrons will go in the 1s orbital. Orbital Diagram Lab Background.
The electron configuration of neutral Na is 1s22s22p63s1 but in Na it loses one electron so it has a new electron configuration of 1s22s22p6 means Na has 268 outermost electrons which makes it stable. The first has been done as an. The electrons are shown as arrows contained in boxes which represents sublevels and orbitals.
Oct 22 2020 Sodium-ion Na means it has lost one electron and has only 10 electrons in the orbitals. The p orbital can hold up to six electrons. Draw the orbital diagram.
Sodium element 11 magnesium element 12 Orbital Diagrams and Valence Electrons. Your electron configuration pattern sheets and the list of ions complete the following table. Give the ground state electron configuration for Se.
Similarly the molecular orbital diagrams for homonuclear diatomic compounds of the alkaline earth metals such as Be 2 in which each metal atom has an ns 2 valence electron configuration resemble the diagram for the He 2 molecule in part c in Figure 493 As shown in part b in Figure 494 this is indeed the case. Problem 59 Hard Difficulty. Tell how many valence electrons the atom has.
Xe6s2 4f14 5d10 6p2. Feb 15 2021 There are 5 valence electrons in Nitrogen Electron Configuration and it lies at the top of group 15 in the periodic table. Tell how many unpaired electrons the atom has.
Nov 10 2017 Use a qualitative molecular orbital energy-level diagram to predict the valence electron configuration bond order and likely existence of the Na2 ion. So Diems and a two plus charge that one Valence electron has been removed. Lithium element 3 beryllium element 4 boron element 5 carbon.
Apr 26 2021 Periodic Table Exceptions To Know. Mar 28 2018 Electron orbital diagrams and written configurations tell you which orbitals are filled and which are partially filled for any atom. Choose the orbital diagram that represents the ground state of N.
Or 3 valence electrons tends to lose all of their valence electrons. Long hand Electron configuration 4. Now many of the users might get confused so the reason is that it can form a bond in the outer.
So the electrons that air left should then follow the noble gas molecular orbital diagram configuration. Using Aufbau Energy Diagrams 2. Sep 11 2020 This is actually the um molecular orbital energy diagram for anything that is a noble gas and sodium although it has one valence electron with two.

Dublin Schools Lesson Orbital Diagrams And Electron Configurations
Https Www Chem Uwec Edu Chem103 F08 F0f Pages Lecture Materials Unit Ii Lecture 8 Publishers Overheads Unit Ii Lecture 8 Publishers Overleads Pdf
Komentar
Posting Komentar